Construction Concerns: Galvanic Corrosion
Article and photos by Greg Havel
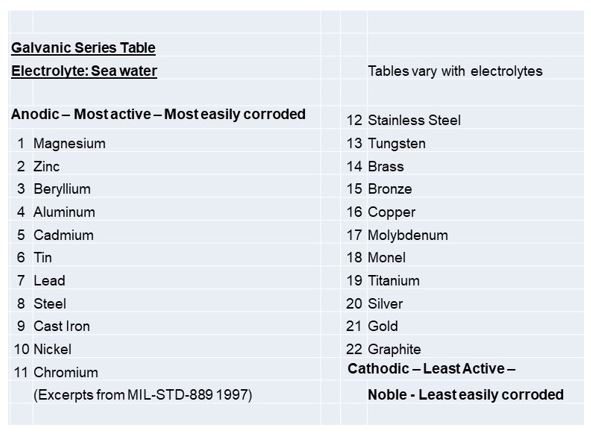
Galvanic corrosion” is damage caused when two dissimilar metals are joined in the presence of an electrolyte. The electrolyte can be one of many liquids, including plain water, acids, sea water, or salt water. When the two metals are joined in the presence of the electrolyte, one becomes the anode and corrodes faster than it would by itself. The other metal becomes the cathode and corrodes more slowly than it would by itself.
This action, the basis for wet-cell and gel-cell batteries, was first demonstrated by Alessandro Volta in 1800.
This action is also the basis for the sacrificial corrosion of one metal to protect another by use of sacrificial anodes or cathodic protection. This sacrificial corrosion is used in the protection of underground steel tanks and steel pipelines, boilers, and water-heating appliances, using magnesium or aluminum sacrificial anodes. This action was first demonstrated by Sir Humphry Davy and Michael Faraday a few years later.
A small electrical current flows between two dissimilar metals in contact in a conductive or corrosive environment. This current flow results in the increased corrosion of the least corrosion-resistant (most active) metal and in the decreased corrosion of the more corrosion-resistant (most inactive) metal. The least corrosion-resistant metal is gradually destroyed by this process, causing weakness and eventually failure of the metal.